Accelerating Medical Device Design with Modeling and Simulation
A conversation with Visa Suomi of MathWorks on how new technology is affecting the medical design workflow.
Latest News
October 23, 2023
Medical orthotic and device design has made huge strides over the past few decades. Recently, however, the capability to design orthotics has accelerated further with the onset of many new technologies related to engineering and digital design as well as methods of fabricating medical products.
Digital Engineering recently spoke with Visa Suomi, Medical Devices Industry Manager at MathWorks, to discuss these trends.
Digital Engineering: What is your perspective on the design trend for patient-specific medical device design? Is medical device manufacturing taking a turn for the better via customization?
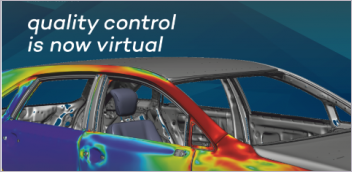
Visa Suomi: Patient-specific medical device designs can be achieved by using computational modeling and simulation (CM&S) techniques such as In Silico Medicine. In Silico Medicine uses virtual human or organ models to adequately replicate their anatomy and physiology (i.e., Digital Twins), that can be then used to validate or clinically evaluate new medical devices in a simulation environment. For example, an electrophysiological heart model could be used to validate pacemakers and other cardiac rhythm management devices, or a virtual kidney model could be used for early feasibility studies for next-generation dialysis machines.
MathWorks sees medical device manufacturers increasingly adopting In Silico Medicine in their R&D due to their benefits accelerating the design process while shortening time-to-market. These In Silico Medicine models help manufacturers to conduct early feasibility studies on their devices without testing on hardware prototypes. Furthermore, these In Silico Medicine models can be tailored towards certain patient populations or groups so that the safety and efficacy of the proposed medical device design can be comprehensively tested with different patient cohorts.
Overall, the use of In Silico Medicine early in the R&D process results in safer and more efficient medical devices for the patients while saving time and costs for the manufacturer. If companies want to remain competitive in the rapidly changing healthcare market, they need to fully leverage the capabilities of computational modeling and simulation in designing new medical devices with tools like MATLAB and Simulink.
Digital Engineering: When it comes to the design of medical devices of all sorts, is the timing ripe for very specific customization, given the advancements of AI as well as other technologies such as 3D printing and additive manufacturing?
Visa Suomi: Yes, the available healthcare data and new technologies such as In Silico Medicine are mature enough to be adopted by the medical device manufacturers. Healthcare providers and device manufacturers are collecting increasing amounts of clinical patient data that can be used to better customize and evaluate next-generation medical devices. Furthermore, many companies are already offering ready-made solutions for Digital Twins of patients/organs (such as validated heart models) that can be used for virtual medical device design and testing. This removes the barrier for the manufacturer to have the knowledge and expertise in creating patient-specific simulation models themselves.
Medical device manufacturers can turn to resources like MathWorks library of In Silico Medicine models that can help to lower the organizational skills and knowledge required to get started with In Silico Medicine.
Digital Engineering: Is there anything else you'd like to add about this topic?
Visa Suomi: There are often concerns from the medical device manufacturers whether computational modeling and simulation (CM&S) techniques are approved by the regulators such as the FDA as regulatory evidence. However, FDA has already published two guideline documents in using CM&S as regulatory evidence: “Reporting of Computational Modeling Studies in Medical Device Submissions” and “Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions”. These guidelines should give enough information for medical device manufacturers to start using these new technologies in their R&D. Furthermore, there are also some industry standards such as the ASME V&V 40 which gives guidance to medical device manufacturers on how to assess the credibility of these simulation models.
More MathWorks Coverage
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
Jim Romeo is a freelance writer based in Chesapeake, VA. Send e-mail about this article to DE-Editors@digitaleng.news.
Follow DE