Customization is enhancing the medical device manufacturing industry, according to one Ricoh expert. Image courtesy of Ricoh.
Latest News
September 22, 2023
Medical orthotic and device design has made huge strides over the past few decades. Recently, however, the capability to design orthotics has accelerated further with the onset of many new technologies related to engineering and digital design as well as methods of fabricating medical products.
The introduction and availability of customized, personalized orthotics and medical devices are further accelerated thanks to many new capabilities facilitating such custom designs: modeling and simulation, digital scanning, 3D printing and additive manufacturing (AM), generative design and artificial intelligence.
This has opened the gateway to a new wave of individualized and customized medical devices, orthotics and products that enhance the lives of many who greatly need them.
With Advance of New Design Tools, The Time is Now
Jason Lichtman is a senior technical specialist for design and manufacturing at Autodesk.
“The future of personalized medical devices is now,” says Lichtman. “Embracing personalized medical device design creates fresh avenues for enhancing patient outcomes, cutting down surgery durations and recovery times and decreasing costs.
“The fundamental ideas behind personalized medical devices are not novel, the convergence of both legacy and cutting-edge technologies empowers companies to manufacture these devices in a more economically viable and adaptable manner,” Lichtman adds. “Leveraging these technologies necessitates overcoming diverse challenges, such as additively manufacturing materials for medical device design, automating the design process and adhering to regulatory requirements.”
Lichtman says that all personalized medical devices are tailored specifically for individual patients; however, there are two primary categories of personalized devices to select from based on the regulatory landscape and the intended purpose.
Bjorn Sjodin is vice president of product management for COMSOL.
Sjodin says that modeling and simulation play an important role in the field of medical device design, offering designers the opportunity to thoroughly examine the safety and performance of a device within a virtual environment, prior to physical testing.
“With patient-specific design, physical testing becomes even more time-consuming and costly,” says Sjodin. “However, a model can easily be adapted and studied using parametric sweeps to simulate the behavior of a wide range of design variants and determine which designs require further testing. Such parametric studies can easily be distributed on a computer cluster to quickly obtain results.
Sjodin adds that simulation with statistical methods such as design of experiments (DOE) and uncertainty quantification (UQ) can be used to narrow down the number of relevant parameter combinations. These methods are also an important part of the verification and validation process; incorporating them into simulations can guide developers to the designs that are most important to include in physical testing. Likewise, regulators can run simulations as part of their approval process.
“In a clinical setting, it might be easy to select a device design based simply on its dimensions and how it physically fits the patient,” he says. “But if the design decision depends on more complex parameters—for example, a device function that involves patient-specific data—a simulation application can be used to find the correct variant for the patient. Created by experts in modeling and simulation, simulation apps can be designed in a user-friendly way, enabling clinicians who lack simulation experience to easily import patient data and execute a personalized simulation at the click of a button.”

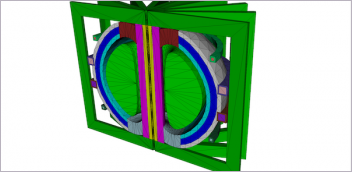
Engineers can use simulation to study radio frequency (RF) heating of implanted devices within MRI systems. The image shows a COMSOL simulation of a human head phantom
in an MRI birdcage coil. Image courtesy of COMSOL and Jim Romeo.
Generative Design Helps with Innovation
The real game-changer in this narrative is generative design, according to Jennifer Samproni, chief technology officer of health solutions at Flex. Similar to how companies in certain industries use algorithms and software to optimize offerings, generative design uses algorithms to create numerous design possibilities that meet specific output requirements.
“Combined with [artificial intelligence (AI)], this technique allows us to generate patient-specific medical device designs based on individual patient data,” Samproni adds. “The ability to test and fine-tune these designs digitally before clinical trials and manufacturing significantly improves the design process and can potentially reduce or eliminate the need for expensive prototypes. As we marry these advancements with the crucial aspect of human-machine interaction (HMI), patient-specific medical device design can reach new heights. HMI ensures that patients and physicians can efficiently use these tailor-made devices, making the customization more than just skin deep.
“Does this evolution signify progress?” Samproni asks. “Without a doubt, yes.”
Yet, Samproni acknowledges, the technology is not without its challenges. The transition to patient-specific design has its hurdles. Take the critical nature of data quality and privacy, for example. When working with sensitive patient information, data quality and privacy have great importance. Also, she notes the implications for cost and accessibility.
“While customization can improve patient outcomes, it might increase prices, making innovative devices less accessible to the most vulnerable populations,” Samproni says. “Navigating through regulatory approval, manufacturing constraints and clinical validation echoes industries’ logistic complexities in delivering custom services across various regions with distinct regulations. Moreover, just as other industries require precision to deliver unique solutions, designing and manufacturing patient-specific devices demands an equally high degree of finesse, and proper training and knowledge for the creators and users of these devices are key.”

“The time of one-size-fits-all treatments is getting behind us. Because of human variability, we are all different and not taking this essential component into account while designing and administering a treatment is negatively impacting the patients.” —Thierry Marchal, Ansys. Image courtesy of Ansys. CLICK HERE FOR FULL-SIZE IMAGE.
Design Flexibility With Additive Technologies
Derek Mathers is the director of clinical applications and business development for AM at RICOH USA. Mathers believes that the design trend for patient-specific medical devices is a significant advancement. Personalized, scalable solutions that ensure an accurate fit can now be created through mass customization with the use of biometric principles and AI algorithms. This trend aligns with industry’s move toward using novel materials and digital fabrication techniques. However, he points out, the genesis of personalized medicine starts within the hospital.

“While innovative technologies have reshaped medical device design, transferring patient data (such as CT or MRI scans) from the hospital to the manufacturer remains archaic, often involving burning CD-ROMs to share DICOM data,” he says. “This represents a significant bottleneck in the creation of patient-match devices.”
To address this challenge, Mathers says the workflow at RICOH 3D for Healthcare integrates the 3D printing process into the hospital system’s radiology viewer, making transfer of 3D patient data much easier.
“Additionally, by situating manufacturing of anatomic models at the point of care, we enable quicker turnaround times and give surgeons more control over their anatomic models,” he says. “The medical device manufacturing industry is turning for the better through customization.” With emerging technologies for design, improved data transfer processes, and advanced manufacturing methods, look for enhanced patient outcomes and an overall efficacy of medical device manufacturing.”
Sachin Misra is a principal with Life Sciences Digital, a Rockwell Automation company. Mishra posits that from a product development perspective, patient-matched devices hold greater appeal as their design and production processes can be optimized and automated. This enables cost-effective mass production with the use of diverse additive and traditional subtractive technologies.
“AM empowers the fabrication of personalized medical devices, incorporating unique features that traditional manufacturing methods cannot achieve,” he says. “These distinctive characteristics not only make personalized medical devices economically feasible but also viable in practice.”

And yet, according to Misra, the restricted availability of AM materials brings a challenge.
“Nonetheless, by employing intelligent design and harnessing the capability of AM to produce intricate structures, this limitation can be successfully surmounted,” he says. “Emerging variations of additive technologies include biologically relevant materials and custom architected materials for specific use cases including osseointegration.”
Misra adds that during the AM design phase, customization expenses can be substantial, which poses another hurdle in creating personalized medical devices.
“The manual design of a personalized device by a skilled engineer can take several hours to a full day, leading to increased costs and longer lead times,” Misra explains. “Achieving a high level of design automation becomes imperative for ensuring the financial viability of personalized medical devices.”
A key aspect of personalized devices development is design automation such as generative design that optimizes the geometry for maximum mechanical biocompatibility while reducing material usage and increasing mechanical strength, according to Misra.
“Empowering design parameters with patient-specific data allows the creation of personalized medical devices tailored to everyone’s distinct physiology,” he says. This data can be achieved through simulations or direct measurements.
AM may be the new frontier for individualized, custom medical device manufacturing now and into the future.
“Creating unique, patient-specific products is faster and easier than ever before,” says Lichtman of Autodesk. “The combination of advances in AM, coupled with advances in CAD automation, have created an environment ripe for patient-specific customization. It is a symbiotic relationship. Advances in AM have pushed software companies to develop tools that can design for AM more quickly and easily, while advances in CAD have enabled designers and engineers to much more easily design geometry so complex that it can only realistically be manufactured via AM.”
As new capabilities emerge each year, the potential of medical devices that are custom fitted to a specific human continues to grow. In medicine, each human being is unique with different sizes, shapes, needs and quirks that are unique to an individual, just as that individual is unique within the world that they live in. As more digital capabilities and engineering design capabilities continue to flourish from the innovation in engineering design, the possibilities are many. Today’s engineering design community and all their capabilities are a game-changer for patients and the medical devices they need and use.
More Autodesk Coverage
More COMSOL Coverage
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
Jim Romeo is a freelance writer based in Chesapeake, VA. Send e-mail about this article to DE-Editors@digitaleng.news.
Follow DE